n3- orbital diagram|N 3 : iloilo Key Takeaways: The electron configuration of nitrogen is [He] 2s2 2p3. The Aufbau principle states that electrons fill the lowest energy orbitals first. Valence electrons are crucial in . 487 Followers, 60 Following, 123 Posts - @pinaywalker on Instagram: "Walk - stop - take out iphone - take a snapshot - resume walking. "
PH0 · N3 lewis structure, Hybridization, Molecular Structure, Bond Angle and
PH1 · N3 lewis structure, Hybridization, Molecular Structure, Bond
PH2 · N 3
PH3 · Molecular Orbitals of the Azide Anion (Physical
PH4 · MO Diagrams for Water and Nitrate Ion
PH5 · Lewis Structure of N3
PH6 · Electron Configuration for Nitrogen (N and N3
PH7 · Electron Configuration For N3
PH8 · 8.3: Electron Configurations
PH9 · 3.3.4: Assembling a complete MO diagram
PH10 · 3.2: Quantum Numbers for Atomic Orbitals
Ge bort Skrapspel till någon du tycker om! Välj bland våra många olika skraplotter och skicka via sms eller e-post. Välkommen till oss på Svenska Spel Tur.
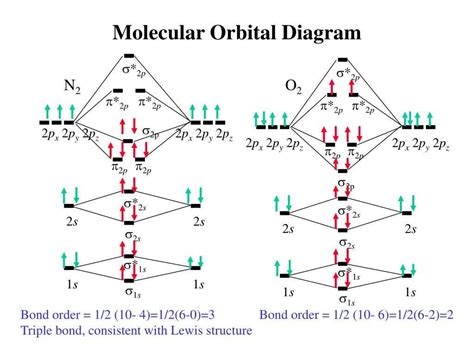
n3- orbital diagram*******Wayne Breslyn. 736K subscribers. Subscribed. 387. 68K views 4 years ago. In this video we will write the electron configuration for N 3-, the Nitride ion. We’ll also look at why Nitrite forms a.A Molecular Orbital Diagram for a diatomic molecule (two atoms) varies in the number of electrons. How do you populate the electrons? Answer • .By Priyanka. October 2, 2021. N3 lewis structure, Hybridization, Molecular Structure, Bond Angle and Shape. The chemical formula N3– represents the Azide ion. The Azide ion is .n3- orbital diagram N 3By Priyanka. October 2, 2021. N3 lewis structure, Hybridization, Molecular Structure, Bond Angle and Shape. The chemical formula N3– represents the Azide ion. The Azide ion is . Oxygen π AO combinations for nitrate ion. You can see that 2 of the combinations don't match the AOs on N, so they will be non-bonding MOs (like 2 lone .Key Takeaways: The electron configuration of nitrogen is [He] 2s2 2p3. The Aufbau principle states that electrons fill the lowest energy orbitals first. Valence electrons are crucial in . University of Florida. CHM2047: One-Semester General Chemistry (Kleiman) 3: Atoms, Orbitals and Electronic Configurations. 3.2: Quantum Numbers for Atomic . Learning Objectives. Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron .
Lewis structure of N3- ion (azide ion) contains two single bonds between each Nitrogen (N) atom. The central nitrogen atom does not have lone pairs, while the outer nitrogen atoms have 2 lone pairs. Let’s . 7.94K subscribers. Subscribed. 100. 5K views 2 years ago #quantum #physics #symmetry. A way of thinking about constructing a qualitative MO diagram for the azide anion. #chemistry.
Step #1: Calculate the total number of valence electrons. Here, the given ion is N3- ion (azide ion). In order to draw the lewis structure of N3- ion, first of all you have to find the total number of valence .Electron orbital diagrams are diagrams used to show the energy of electrons within the sublevels of an atom or atoms when used in bonding. Single atom diagrams (atomic orbital diagrams) consist of horizontal lines or boxes for each sublevel. Within orbitals, arrows indicate the spin direction of the occupant electrons.
Explain with the help of the orbital diagrams, why the 2p-subshell of nitrogen is more stable than that of oxygen. The electronic configuration of nitrogen (N) is 1s 2 2s 2 2p 3. The orbital diagram .
The allowed values of l depend on the value of n and can range from 0 to n − 1: Equation 2.5.2 l = 0, 1, 2,., n − 1. For example, if n = 1, l can be only 0; if n = 2, l can be 0 or 1; and so forth. For a given atom, all wave functions that have the .n3- orbital diagramOrbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.
An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and down (ms = -1/2). Azide [N3]- ion Lewis structure, molecular geometry or shape, resonance structure, polar or non-polar, hybridization, bond angle. N 3– is the chemical formula for the azide ion, also known as hydrazoate. It is an anion composed of three nitrogen (N) atoms. It is the conjugate base of hydrazoic acid/ hydrogen azide (HN 3 ).
N 3See Answer. Question: Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−. Use the buttons at the top of the .
An s orbital is not affected when the atom at one end of the bond is rotated with respect to the other. A p orbital is affected by such a rotation. If one atom turns with respect to the other, the p orbital would have to stretch to maintain the connection. The orbitals would not be able to overlap, so the connection between the atoms would be lost. 2. N3– lewis structure octet rule: A N 3 – ion has eight valence electrons in total. N has 5 valence electrons, and its configuration is 1s 2 2s 2 2p 3, therefore N-3 has 3 additional electrons, and its octet is complete. 3. N3– lewis structure resonance: The azide ion has resonance structures that may be calculated. These are displayed .

Electron configurations have the format: 1s 2 2s 2 2p 6 . The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same .The four chemically important types of atomic orbital correspond to values of ℓ = 0 ℓ = 0, 1 1, 2 2, and 3 3. Orbitals with ℓ = 0 ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 n > 1 and ell = 0 e l l = 0 contain one .The following equation summarizes these relationships and is based on the hydrogen atom: ΔE = Efinal − Einitial = − 2.18 × 10 − 18( 1 n2 f − 1 n2 i)J. The values nf and ni are the final and initial energy states of the electron. The principal quantum number is one of three quantum numbers used to characterize an orbital.Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau. Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic .write orbital diagram for N3- in order of increasing energy statrting at the bottom with the lowerst energy orbitals. Orbital Diagram of elements: Electronic configuration is the distribution of electrons in the atomic orbitals like s,p,d,f,g. It is a scientific notation which follows many rules like Aufbau Principle, Pauli's Exclusion . 3.2: Quantum Numbers for Atomic Orbitals is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Schrödinger’s approach uses three quantum numbers (n, l, and ml) to specify any wavefunction. The quantum numbers provide information about the spatial distribution of an electron.
Too bad I can't make the edits like you can, thanks for the Hawkgirl edit and your Huntress attempt! The cloak itself wasn't the biggest problem, just the fact that detail on her arm was partially obscured, making her gloves unusable This better?
n3- orbital diagram|N 3